Research Corner: The Complexity of Ventilation-Limited Fires
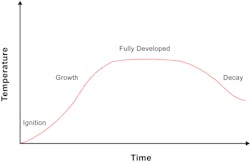
Results of the UL Fire Safety Research Institute (FSRI) DHS 2008 grant, “Impact of Ventilation on Fire Behavior in Legacy and Contemporary Residential Construction,” challenged the widely accepted fire growth curve that is found in many of the prominent fire service training manuals.1 The project uncovered that fires burning inside a structure followed a different fire growth curve when compared to fires burning outside a structure. The results indicated that the traditional fire growth curve, shown in Figure 1, neglected the effect of oxygen depletion.
When the report was released in 2010, this concept seemed to be missing from many of the discussions on fire dynamics and even fire service training. Although the results are not viewed as a new concept or a major scientific discovery, this project shed new light on the effects that compartmentation plays in fire growth and development.
One of the fundamental educational tools used to demonstrate fire dynamics in the fire service is the fire tetrahedron. It graphically identifies the four ingredients needed to support combustion: heat, fuel, oxygen and a chemical chain reaction. Each ingredient is independent from the other, and removing or slowing any of these four extinguishes the fire.2 Taking it one step further would be to say that limiting any of these four would limit the size of the fire. When a fire is located within a compartment or structure, provided enough fuel is available, the fire size will be limited based on the amount of available oxygen.
Testing the theory
In order to validate this, UL FSRI used the scientific principal known as Thornton’s Rule. Originally published in 1917, Thornton’s Rule states that the heat released per unit of oxygen consumed was fairly constant for the complete combustion of a large number of organic gases and liquids.3 Thus, if the amount of oxygen consumed could be measured, the heat release rate of the fire could be approximated. This technique, known as oxygen consumption calorimetry, has been refined since 1917 to allow the accurate measurement of the energy released from any burning object or room. The oxygen consumption measurement system at the UL Large Fire Lab in Northbrook, IL, (Figure 2) collects the combustion products and determines the amount of oxygen consumed. The hood can accurately measure up to a 10-MW fire.
FSRI conducted two room-scale experiments under the oxygen consumption calorimeter—one with typical living room/family room furnishings (Figure 3) and one over-furnished living room (Figure 4). The intent was to measure the energy released by the fire over time and verify if the fire size was being controlled by the available oxygen or available fuel.
The rooms were ignited in the corner of the rear sofa and permitted to grow unchecked until the fuel was consumed or the enclosure failed. In each experiment, the total heat release rate versus time was recorded using the oxygen consumption technique. Figures 5 and 6 show the furnished and over-furnished rooms over time as the fires progressed, reached their peaks, before they began to decay. Figure 7 shows the energy release over time for both rooms.
Results and analysis
The furnished compartment reached a fully developed stage within 3 minutes of ignition and maintained that heat release rate for 3 minutes before it began to decay. The over-furnished compartment reached a similar peak heat release rate at just under 6 minutes and maintained that through the failure of the ceiling at 10 minutes, at which time it increased. The over-furnished compartment had more fuel to consume, thus, it remained fully developed until the test was extinguished. Figure 7 shows the heat release rate and total heat released for both compartments.
The difference in the two growth rates can be attributed to the orientation of the fuel in the compartment. The addition of the curtains in the furnished compartment sped up the growth, as they were located above the ignition point and promoted flame spread vertically. Although the growth rates were different, the rooms both reached a peak heat release rate of approximately 6.5 MW.
The rooms were the same size and had the same ventilation opening. The only difference was the fuel load. With six times as many chairs and three times as many sofas, the over-furnished room reached the same heat release rate as the furnished compartment with a single sofa and single chair. The energy release or size of the fire was limited by the ventilation opening. The opening was just over 70 ft2. Based on the experiments, maximum fire size for this ventilation opening was approximately 6.5 MW. At its peak, only so much oxygen could interface with fuel for combustion.
In the over-furnished compartment, as portions of the ceiling began to fail after 10 minutes, more oxygen was able to interact with fuel and the size of the fire increases to 8 MW and then 10 MW. This demonstrates how providing additional ventilation to a ventilation-limited fire will increase the energy release rate of a fire.
The fact that the furnished compartment looks very similar to Figure 1 is because once the fire grew to its maximum state, no additional ventilation was provided and no exposures were present. With enough time, and no exposures, the fire was able to run out of fuel, entering a fuel-limited decay stage. With the size of the ventilation opening in this case, the fire entered this stage approximately 4 minutes after reaching its peak. With a smaller opening, such as a single door or single door and common residential-size window, this time would have been longer.
In contrast, the over-furnished compartment was provided with additional ventilation, be it unplanned, when the ceiling began to fail. This additional ventilation increased the fire size. The much larger fuel load resulted in the fire maintaining its ventilation-limited state for over 7.5 minutes at which time the experiment was ended. At the time of extinguishment, the fire was still in its ventilation-limited state.
The concept of a fire being limited by the available oxygen is not only applicable to a compartment but also a structure. Simply put, a structure is a series of compartments or rooms joined together with openings between. The compartments have a defined fuel load and ventilation profile. A fire located in any one compartment will consume the available oxygen in its compartment of origin and any adjacent compartments connected by openings such as open doors, provided there is sufficient fuel. Once this has occurred, the fire becomes limited by the available ventilation provided from open windows or doors and natural leakage in the exterior walls. Additional ventilation, such as unplanned ventilation (e.g., window failure or tactical ventilation conducted by fire crews), has the potential to provide additional oxygen and increase the size of the fire. Any increase in the size of the fire will increase the thermal exposure to firefighters or victims inside the structure as well as increasing the toxic gasses produced.
Reviewing the heat release rate data from Figure 7 even further, we see the possibility of ventilating a structure enough to make the fire limited by the fuel available is difficult. The compartment in the experiment had an opening area of 70 ft2, which resulted in ventilation-limited fire conditions with a single sofa and single chair. A single door may have an area of 21 ft2, a large window 15 ft2–20 ft2. Even openings as large as a sliding glass door may have a clear opening of 42 ft2. Just to match the opening size on the compartment tested, fire crews would need to preform tactical ventilation of a combination of a sliding door, single door and a large window. If the fuel load was a typical living room setup, even with this 70 ft2 of ventilation, the fire would still be ventilation-limited. Most compartments in residential structures have less potential ventilation points in a single compartment, making it nearly impossible to provide enough ventilation to result in a fuel-limited fire.
The fire growth curve in Figure 8 shows the potential complexities of a room-and-content fire within a residential structure. Rather than five defined stages, like in Figure 1, the ventilation-limited curve is more complex. Understanding the concept of ventilation-limited fires helps incident commanders, line offices and firefighters identify the potential fire growth or decay as a result of the wide variety of tactics.
In sum
Reviewing these two compartment experiments can enhance our understanding of why a fire is ventilation-limited, and how tactical choices have the potential to change the fire’s size. The furnishings found in residential structures have the potential for rapid fire growth, resulting in fire service intervention more often than not occurring during a point where the fire is in a ventilation-limited state. Adapting to the changing fire environment by understanding fire dynamics allows for safer and more effective fireground operations.
References
1. S. Kerber, “Impact of Ventilation on Fire Behavior in Legacy and Contemporary Residential Construction”. Underwriters Laboratories. Northbrook, IL. December 2010.
2. F. Stowell, “Essentials of Firefighting 6th Edition”. International Fire Service Training Association. Stillwater, OK. January 2013.
3. W. Thornton, “The Relation of Oxygen to the Heat of Combustion of Organic Compounds,” Philosophical Magazine and J. of Science, Vol 33, No. 196, 1917

Robin Zevotek
Robin Zevotek is a research engineer with the Underwriters Laboratories (UL) Firefighter Safety Research Institute and a captain with the Ellicott City Volunteer Fire Department in Howard County, MD. He holds a master’s degree in fire protection engineering from the University of Maryland and is a licensed professional engineer (PE).