ZOLL X Series Monitor/Defibrillator Receives 510(k) Clearance From FDA
ZOLL Medical Corporation, a manufacturer of medical devices and related software solutions, has received 510(k) clearance from the U.S. Food and Drug Administration to market and begin U.S. distribution of the new ZOLL X Series™ Monitor/Defibrillator. Designed primarily for EMS operations, the X Series is about half the size and half the weight of competitive full-featured monitor/defibrillators, yet much more powerful and built to the most extensive standards for ruggedness.
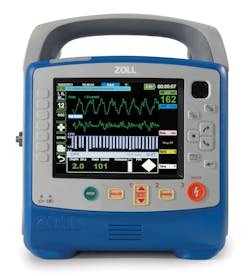
Weighing less than 12 pounds, the X Series is compact without compromise in capability, performance, or display size. This monitor/defibrillator combines the clinically superior therapeutic capabilities of ZOLL defibrillation, pacing, and CPR assistance with advanced monitoring parameters.
“We believe the X Series is a game changer for ZOLL in the EMS market. We have the size and weight advantages that ZOLL is known for without compromising features or functionality,” said Jonathan A. Rennert, President of ZOLL. “The X Series has every advanced monitoring and communication capability required by EMS providers and, ultimately, critical care transport operations.”
“The X Series should be a ‘must see’ for any service interested in cutting edge technology. It was built on the Propaq® MD Monitor/Defibrillator platform, which in little more than a year has become a standard of care for air medical operations worldwide,” he added.
The large bright screen on the X Series allows for simultaneous viewing of up to four different waveform traces, as well as split-screen 12-lead views. The device incorporates Masimo rainbow® SET CO-Oximetry™ technology, which provides Masimo SET® SpO2, pulse rate, perfusion index (PI), carboxyhemoglobin (SpCO®), and methemoglin (SpMet®). Welch Allyn Non-invasive Blood Pressure (NIBP) technology-Sure BP® and Smart Cuf®-is yet another capability that sets the X Series apart from other defibrillators. Acknowledged as the best of class NIBP technology, it offers faster, more accurate readings.
For unequaled CPR support, the X Series features ZOLL’s full suite of CPR assist tools including Real CPR Help® for real-time CPR feedback and See-ThruCPR® artifact filtering to help minimize the length of pauses. It’s the first device to feature ZOLL’s CPR Dashboard™, which displays CPR quality measures in real time.
A major breakthrough in the X Series is its advanced communications capabilities. Leveraging a dedicated onboard data processor—essentially a smart phone in the box—the X Series is the first and only EMS monitor/defibrillator that can communicate via Bluetooth, WiFi, or USB cellular modem. Streaming data can be sent to hospitals or clinicians at remote locations while rescuers are caring for patients. For STEMI patients, the X Series is designed to speed door-to-balloon times with quick and accurate 12-lead acquisition, interpretation, and transmission. The X Series fully integrates with ZOLL’s RescueNet® 12-Lead and RescueNet Medgate Management Systems.
The X Series, along with the Propaq MD, are the only two defibrillators to earn an IP55 ingress protection rating for dust and water. Both units are virtually immune to a three-minute, high-pressure onslaught of water and meet the most stringent specifications for shock and vibration. The X Series is powered by a SurePower™ II high-capacity lithium-ion battery for six hours of continuous run time.