VAPOR PRESSURE
Vapor pressure of a liquid is defined in the Condensed Chemical Dictionary as "the characteristic at any given temperature of a vapor in equilibrium with its liquid or solid form. This pressure is often expressed in millimeters of mercury, mm Hg." In simple terms, vapor pressure is the pressure being exerted by the liquid against atmospheric pressure. When the pressure of the vapor is greater than atmospheric pressure, vapor will spread beyond an open container or an open spill. If the pressure of the vapor is less than atmospheric it will be present only over and near the surface of the liquid. If liquid is in a container, vapor pressure is the pressure being exerted by the liquid vapors on the container. For example, when a gas has been liquefied, the only thing keeping it a liquid is the pressure in the tank. The liquid is already above its boiling point. The pressure inside the container is the atmospheric pressure in that container. It can be much higher than outside atmospheric pressure. For example, if the pressure in the tank is 50 psi, the atmospheric pressure in that tank is 50 psi regardless of conditions outside the tank. Boiling point is related to vapor pressure and vapor content although the relationship is opposite in nature. Materials with low boiling points and flash points will have high vapor pressure and high vapor content.
VAPOR CONTENT
Vapor content is the amount of vapor that is present in a spill or open container. The lower the boiling point and the flash point of a liquid, the more vapor there will be. The parallel relationship of boiling point and flash point is comparable to the opposite relationship of vapor pressure and vapor content in the diagram below, using a seesaw to illustrate the up-and-down and opposing relationship.
As shown in the previous illustration, when the boiling point and flash point are low, vapor content and vapor pressure are high. When boiling point and flash point are high, vapor pressure and vapor content are low. The lower the boiling point or flash point of a liquid, the higher the vapor-content at a spill and the higher the vapor pressure inside a container. If a liquid is above its boiling-point temperature, there is likely to be more vapor moving farther away from a spill. If the liquid is below its boiling-point temperature, there will be some vapor above the surface of the liquid, but it will not travel very far. If the flammable liquid in a container is below its boiling point, the vapor content and vapor pressure in the container will be low. If the flammable liquid in a container is above its boiling point, the vapor content and vapor pressure in the container will be high.
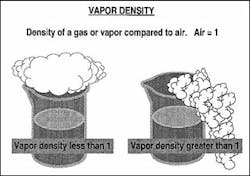
VAPOR DENSITY
Vapor density is a physical characteristic that affects the travel of vapor; it is the weight of a vapor compared to the weight of air (Graphic #2). Vapor density is usually determined in the reference books by dividing the molecular weight of a compound by 29, which is the assumed molecular weight of air. Air is given a weight value of 1, which is used to compare the vapor density of a material. If the vapor of a material has a density greater than 1, it is considered to be heavier than air. Heavier-than-air vapor will lie low to the ground and collect in confined spaces and basements. This can cause problems because many ignition sources are in basements, such as hot-water heaters and furnace pilot lights. If the vapor density is less than 1, the vapor is considered to be lighter than air, so it will move up and travel farther from the spill.
VOLATILITY
Another term associated with vapor is volatility. It is the tendency of a solid or a liquid to pass into the vapor state easily. This usually occurs with liquids that have low boiling points. A volatile liquid or solid will produce significant amounts of vapor at normal temperatures, creating an additional flammability hazard. The vapor produced by a volatile liquid is affected by wind, vapor pressure, temperature, and surface area. Temperature always causes an increase in vapor pressure and vapor content in an incident. The more vapor pressure in a container the greater chance of container failure. The more vapor content, the farther the vapor may travel away from a spill.